Electron Transport Chain
- The electron transfer chain is embedded in the inner membrane of the mitochondria and is known as the resispiratory chain.
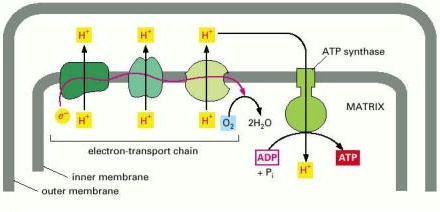
- The electron carriers NADH and FADH generated in the citric acid cycle transfer the electrons gained from the oxidation steps, to the electron transport chain.
- The first step is the removal of the hydride ion from the NADH, which generates a proton (+H) and two high energy electron.
- The electrons then pass along the chain of specialized donor and acceptor molecules. The electrons fall to a lower energy level as they move along the chain. The energy released is then used to pump hydrogen (H+) atoms across the inner membrane, from the matrix to the intermembrane space. This thereby generates a hydrogen gradient that serves as a source of energy.
Image courtesy of www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=mboc4&part=A163#A304
Movement of hydrogen ions across membrane.
- The movement of hydrogen ions generates a pH gradient and a voltage gradient across the inner mitochondrial membrane. The pH in the matrix is much higher than in the cytosol. The pH gradient drives H+ back into the matrix and OH- back out which reinforces the potential gradient, with the inside negative and the outside positive.
- The pH and the voltage gradients together form the electrochemical proton gradient.
- The electrochemical proton gradient exerts a proton-motive force, which is measured by millivolts (mV). It is about 200 mV across the inner membrane of respiring mitochondrion.
Synthesis of ATP
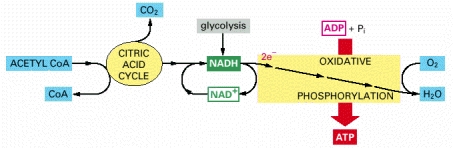
- Most of the energy is harnessed from the electron transfer is then used for the generation of ATP from the phosphorylation of ADP, termed oxidative phosphorylation.
- The electrochemical proton gradient is used to drive the ATP synthesis and uses the membrane bound enzyme ATP synthase.
- This enzyme is composed of a ring of 6 subunits which projects on the matrix side of the inner mitochondrial membrane.
- The ATP synthase provides a hydrophilic pathway across the inner membrane to allow the passage of protons move down their electrochemical gradients. The movement of the protons through the ATP synthase is used to drive the reaction of ADP and P to make ATP.
- This occurs by the stator-rotor protein structure located in the ATP synthase in which the passage of protons causes the rotor ring to spin. The rotor is also attached to stalk which causes it to spin too. The stalk spins rapidly inside a large enzymatic structure of the ATP synthase, known as the lollipop head.
- The flow of protons down the concentration gradient causes the two sets of proteins to rub against each, which generates mechanical energy. ATP is generated by the repeated conformation change that is produced by the rotating stalk. The mechanical energy is converted into chemical bond energy to form the ATP molecule.
- ATP synthase is able to generate more than 100 ATP molecules per second and about three to four protons are required to make each ATP molecule.
- At the end of the series of transfers, the electrons are at their lowest energy levels. They combine with oxygen and also with protons to produce molecules of water.
Image courtesy of www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=mboc4&part=A163#A304